A robust portfolio
A novel pipeline
Program
Discovery
Preclinical
Phase 1
Phase 2
Phase 3
Next Milestone
Antibody-Drug Conjugate (ADC)
Discovery
Preclinical
Phase 1
Phase 2
Phase 3
Phase 1 Part 1
Prelim Data Fall 2024
Immuno-Oncology (I/O)
Discovery
Preclinical
Phase 1
Phase 2
Phase 3
Discovery
Preclinical
Phase 1
Phase 2
Phase 3
Paused
Partnered Programs
Molecule*
Licensee
Therapeutic Area
Preclinical
IND-Enabling
Phase 1
Phase 2
Phase 3
Out-Licensed
BD0801

Ovarian cancer
Preclinical
IND-Enabling
Phase 1
Phase 2
Phase 3
9MW0211

Ocular disease
Preclinical
IND-Enabling
Phase 1
Phase 2
Phase 3
TRK-950

Oncology
Preclinical
IND-Enabling
Phase 1
Phase 2
Phase 3
Antibody-Drug Conjugates (ADCs)
ADCs are validated, powerful tools in the cancer treatment armamentarium that offer a number of potential benefits over traditional cytotoxic cancer treatments, such as targeted drug delivery, improved efficacy and lower toxicity. Advanced technologies are enabling the creation of a next generation of ADCs with improvements in several key components, including linkers, conjugation chemistries and payloads. By using our innovative capabilities, Pyxis Oncology is uniquely positioned with an end-to-end system for creating novel, next-generation ADC approaches to create candidates with better stability to limit early payload release prior to reaching the tumor, a more consistent drug product and more homogeneous drug/antibody ratio (or DAR), and more potent and permeable payloads – all of which together may lead to better tolerated, more active therapies for patients.
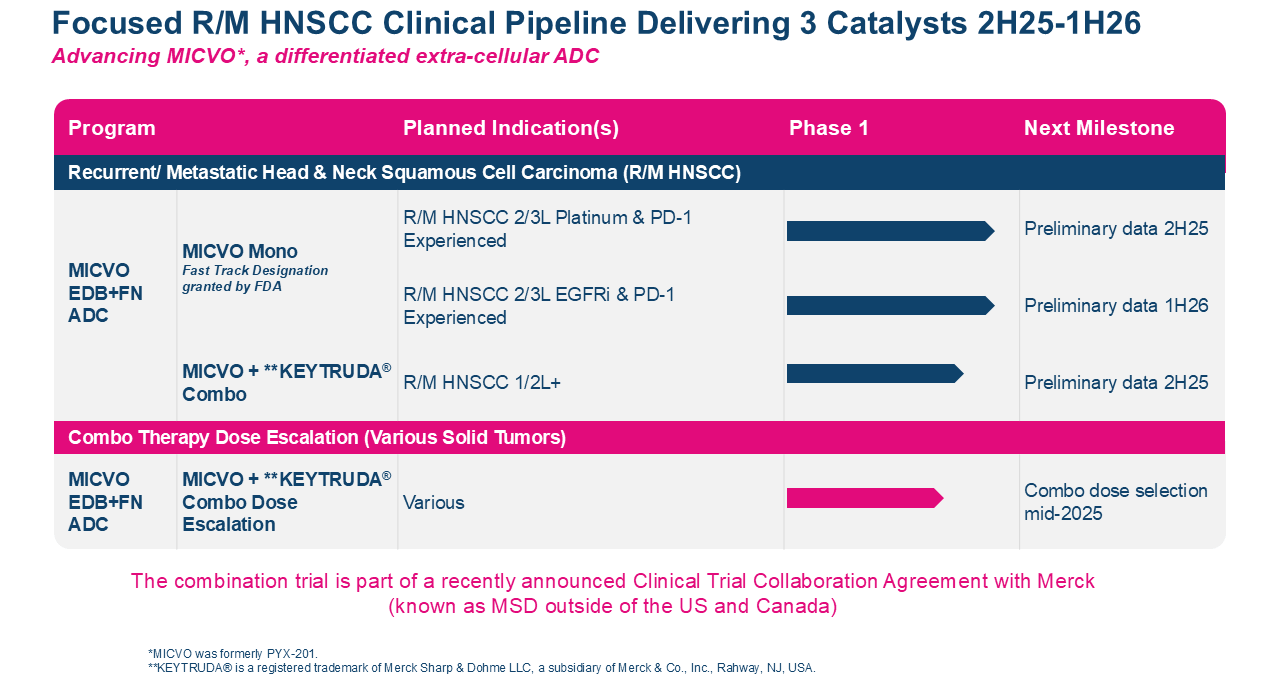
PYX-201
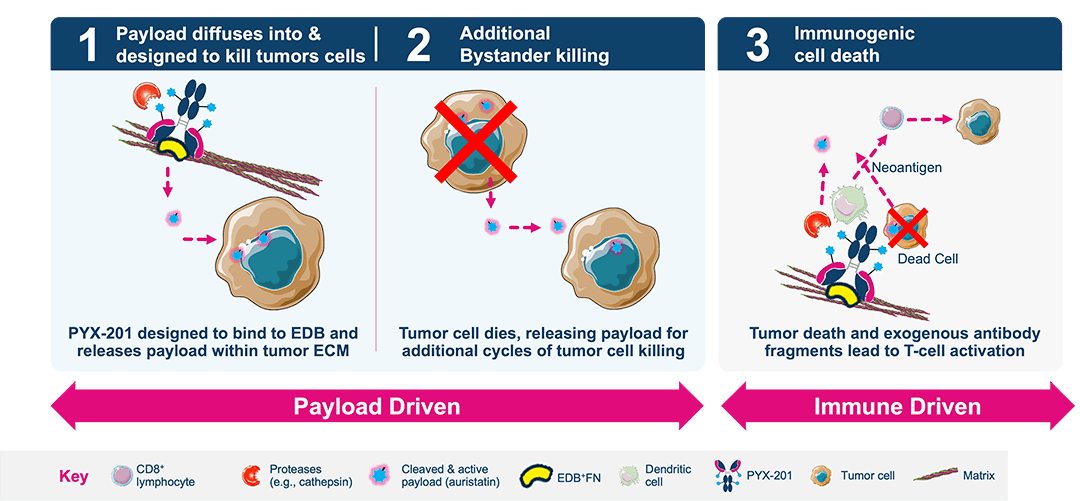
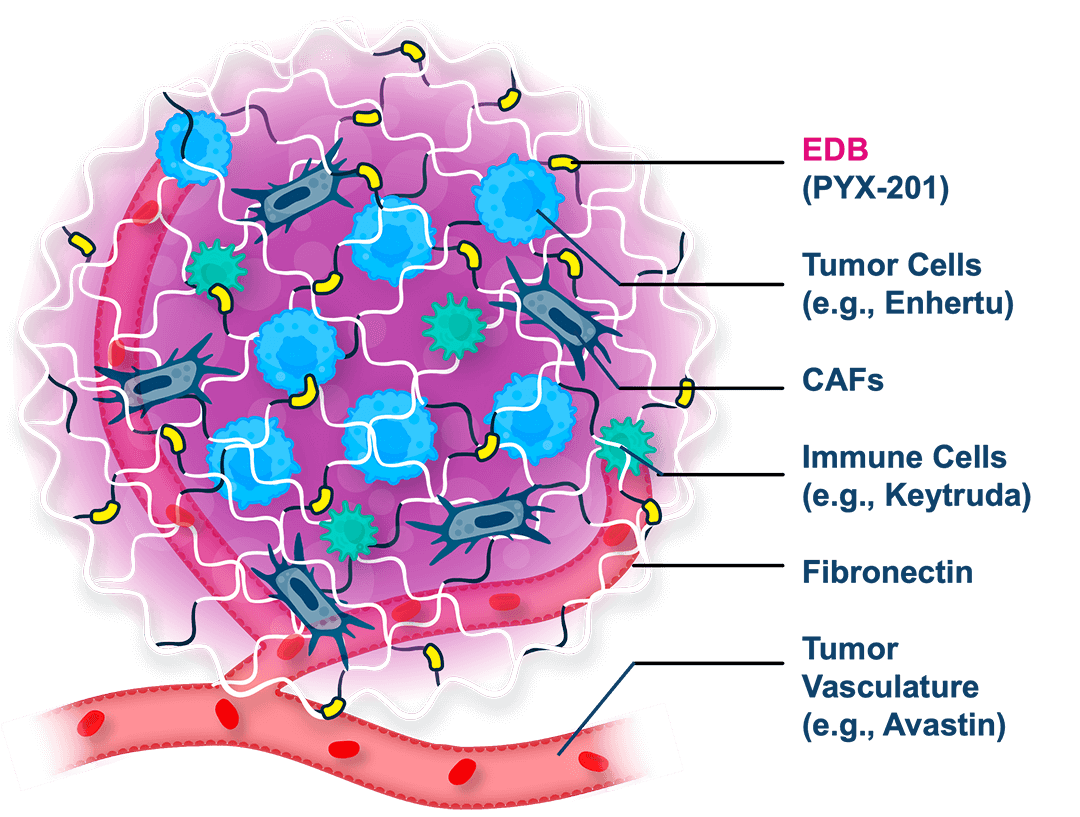
A first-in-concept ADC targeting a noncellular structural component of the extracellular matrix (ECM) with a multipronged mechanism of action
PYX-201 is an antibody-drug conjugate (ADC) that binds to extradomain-B (EDB) fibronectin, an insoluble integral component of the noncellular antigen that is overexpressed in many malignancies and minimally expressed in most normal adult tissues. After binding, its highly cell-permeable and improved auristatin derivative payload is enzymatically cleaved and released extracellularly by proteases in the tumor microenvironment, presumably diffusing into and killing nearby tumor cells and cells that form the supportive tumor infrastructure. PYX-201 can also elicit an antitumor immune response by recruiting T cells and stimulating dendritic cell maturation. While its effects are primarily due to its activity targeting extracellular noncellular antigen in the ECM, a fraction of the PYX-201 toxic payload may also be internalized, further enhancing its antitumor activity.
PYX-201 is currently being investigated in an open-label, multicenter, Phase 1 dose-escalation trial in patients with relapsed or refractory solid tumors. The U.S. Food and Drug Administration (FDA) granted Orphan Drug Designation (ODD) for PYX-201 in pancreatic cancer. Learn more about the PYX-201-101 trial here.
Opportunistic Immuno-oncology (I/O)
We are building a diverse portfolio of immunotherapies that target broad immune regulatory mechanisms as well as novel immune checkpoints with the potential to transform the cancer treatment landscape. Our approach draws from externally developed programs and our internal discovery engine based on the work of Pyxis Oncology’s scientific founder.
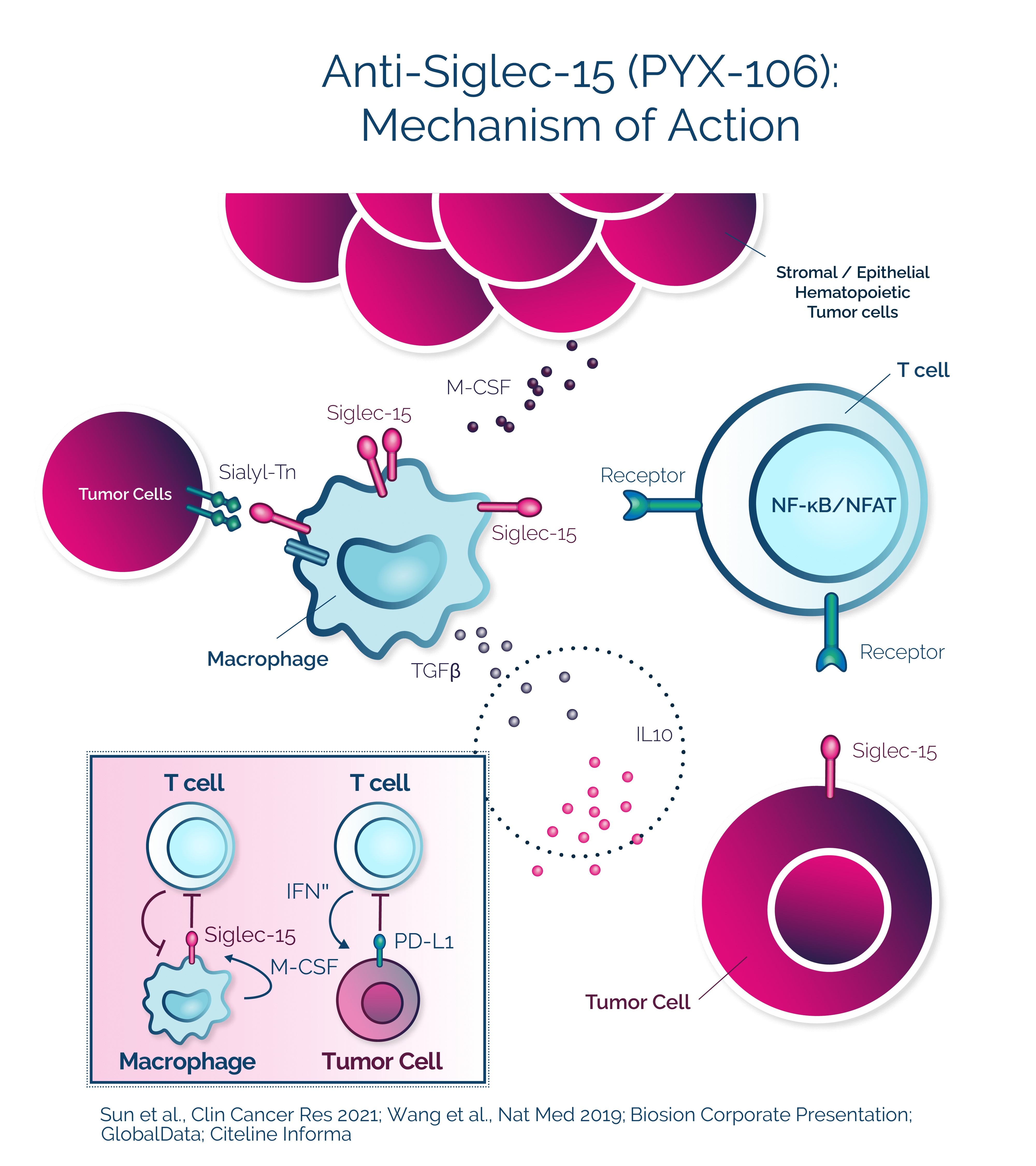
PYX-106
A new immune checkpoint inhibitor that may be complementary to PD-1/PD-L1 targeted therapies
PYX-106 is a fully human monoclonal antibody designed to target Siglec-15, an immune suppressor broadly expressed across different tumor types and on macrophages. PYX-106 could potentially address PD-1/PD-L1 non-responders across a variety of tumor types and may be integral in combination with other cancer therapies.
PYX-106 demonstrated a ten-fold greater binding affinity to human Siglec-15 and higher potency in activating T cells compared to current benchmarks in development, which could potentially lead to better activity and more flexible dosing.
PYX-106 is currently being investigated in an open-label, multicenter, Phase 1 dose-escalation in patients with advanced solid tumors. Learn more about the PYX-106-101 trial here.
Paused Programs
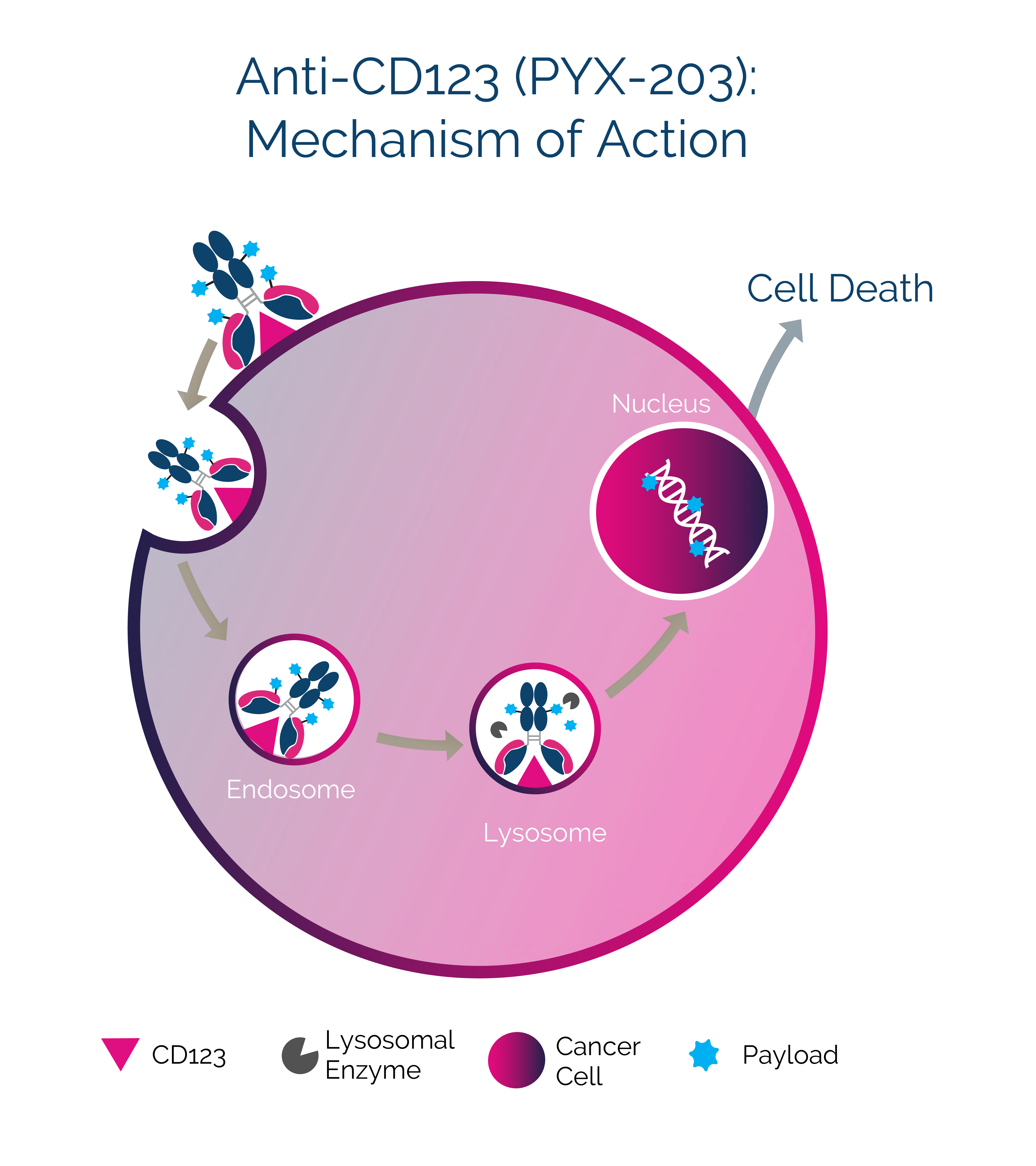
PYX-203
A superior ADC for the treatment of hematologic cancers
PYX-203 targets and binds to the interleukin-3 receptor, also known as CD123, a rapidly internalizing target that is overexpressed in hematologic cancers by leukemic blasts and stem cells. After internalization, its highly potent cyclopropylpyrroloindoline (CPI) payload is enzymatically released and trafficked to the nucleus, where it crosslinks DNA. CPI is engineered for enhanced tolerability and may allow PYX-203 to reach a broader patient population. CPI is resistant to drug efflux pumps and could confer superior cancer-killing activity. The antibody is also engineered to have a modified Fc region to mitigate off-tumor toxicity.
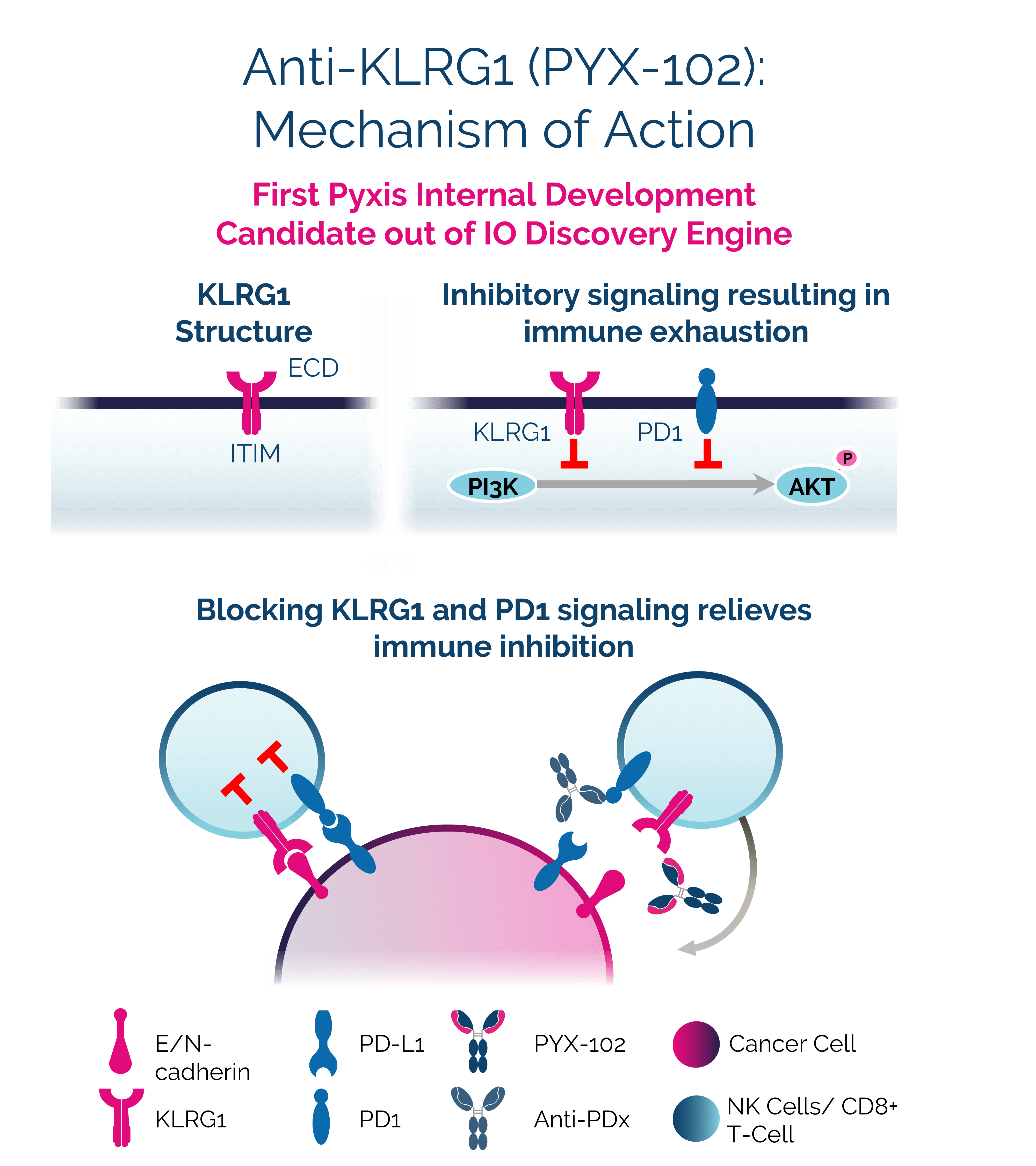
PYX-102
An I/O strategy to relieve immune inhibition and activate an antitumor response
PYX-102 targets killer cell lectin-like receptor subfamily G member 1 (KLRG1), an inhibitory receptor expressed on T cells and natural killer (NK) cells. Its ligands, E- and N-cadherin are expressed in numerous solid cancers. By blocking KLRG1 signaling, PYX-102 may relieve immune inhibition in these tumors while rescuing KLRG1-mediated suppression of human CD8+ T cells. PYX-102 has significant potential as a monotherapy and in combination treatment strategies.
PYX-107 (sotigalimab)
A new approach in immuno-oncology
Sotigalimab uniquely binds and activates CD40 and has been engineered to be more potent with a more favorable safety profile. These design features may enable sotigalimab to overcome the dosing limitations of other anti-CD40 approaches and be the best-in-class CD40 agonist.
Sotigalimab has been evaluated in more than 500 patients in clinical trials and demonstrated strong anti-tumor activity, including rapid, deep, and durable responses and a favorable tolerability profile across multiple difficult-to-treat tumor types.
Currently, sotigalimab is being evaluated in an investigator-initiated Phase 2 trial in combination with doxorubicin in patients with advanced soft tissue sarcoma (STS) and interim data has demonstrated OS improvement in all STS patients treated with sotiga and doubling of PFS in dedifferentiated liposarcoma (DDLPS), a rare subtype of liposarcoma. Learn more about the trial here. Sotigalimab has also been evaluated in a multicenter, open-label Phase 2 trial in combination with nivolumab in patients with PD-(L)1 blockade refractory melanoma. Learn more about the trial here.
APX601
Anti-TNFR2 Antagonist mAb
APX601 is a novel TNFR2 antagonist antibody designed to reverse immune suppression in the TME and unleash immune-mediated tumor killing activity through a unique mechanism of action. APX601 may deplete and inactivate TNFR2-expressing Tregs, reverse myeloid-mediated T cell suppression and directly kill TNFR2-expressing tumor cells. In preclinical models, APX601 has demonstrated anti-tumor activity and may have applicability for the treatment of multiple tumor indications of unmet medical need. APX-601 was obtained as part of Pyxis Oncology’s acquisition of Apexigen in August 2023 and its development was previously paused by Apexigen.
APX801
Natural killer (NK) cell engager
APX801 is an NK cell engager designed to specifically activate natural killer cells leading to effective killing of tumor cells currently at the pre-clinical stage. APX801 was obtained as part of Pyxis Oncology’s acquisition of Apexigen in August 2023 and its development was previously paused by Apexigen.

Immuno-oncology (I/O); Antibody-Drug Conjugate (ADC)