Our platform-based approach
Flexible Antibody Conjugation Technology (FACT) Platform
ADCs consist of three key components: an antibody, a payload, and a linker. Bringing these 3 components together in a safe and effective drug is a challenge for ADC development.
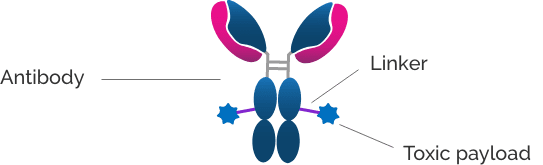
ADCs are validated, powerful tools in the cancer treatment armamentarium that offer a number of potential benefits over traditional cytotoxic cancer treatments, including targeted drug delivery, improved efficacy and lower toxicity.
Advanced technologies, including those we have from our FACT platform, are enabling the creation of a next generation of ADCs with improvements in three key areas – linkers, conjugation chemistries and payloads.
By using our innovative capabilities, we aim to improve upon earlier generation ADC approaches to create candidates with better stability to limit early payload release prior to reaching the target, a more consistent drug product, more homogeneous drug/antibody ratio (or DAR), and more potent and cell-permeable payloads – all of which together may lead to improve the therapeutic window to maximize an ADC’s clinical benefits for patients.
APXiMAB Platform
Our antibody discovery platform, APXiMAB, is designed to create diverse antibodies with high affinity, specificity, and stability to use as the backbone for antibody-drug conjugates (ADCs) and to generate immunotherapy candidates. APXiMAB is a validated antibody discovery platform that has produced several clinical candidates in addition to one marketed product, which provides a royalty stream.
APXiMAB leverages proprietary rabbit fusion cell lines, with unique biology not captured by traditional approaches, along with MLG humanization technologies that enable the discovery of candidates with high affinity against various targets, including those that are difficult to drug with conventional technologies.
By combining the powerful APXiMAB platform for antibody discovery and our flexible antibody conjugation technology (FACT), we are uniquely positioned with an end-to-end system for creating novel, next-generation ADCs.
Our Approach to ADCs
ADCs deliver potent cancer-killing agents directly to tumor cells. Our technology platform is designed to enable us to develop safer and more effective ADCs.
Our Immuno-oncology (I/O) Target Catalog
Over the past decade, I/O therapeutics have brought a unique approach to cancer treatment.
However, tumor response rates remain low for many patients, particularly for tumors with low levels of tumor-infiltrating lymphocytes (TILs). These non-inflamed (i.e., “cold”) tumors can suppress the immune response through a variety of mechanisms.
Using deep bioinformatics analyses, preclinical research, and human biomarker analysis to understand the role of TILs in cold tumors, we have identified broad immune regulatory mechanisms as well as novel immune checkpoints that could serve as the foundation for new immunotherapies.
Our Approach to I/O
Pyxis Oncology is building a diverse portfolio of immunotherapies that target broad immune regulatory mechanisms as well as novel immune checkpoints.
