Clinical Programs
Clinical trials
Pyxis Oncology is advancing breakthrough therapeutics with the potential to defeat difficult-to-treat cancers.
Our lead candidate, micvotabart pelidotin (MICVO, formerly PYX-201), is currently being evaluated in Phase 1 clinical trials across various solid tumors. Promising results in recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) are guiding our focus on developing effective treatments for these patients.
Find a trial site near you
Refer your patients to an active trial site near you.
Our study is enrolling at multiple locations nationwide.
Lead program
Our lead therapeutic candidate: MICVO
Micvotabart pelidotin (MICVO, formerly PYX-201)
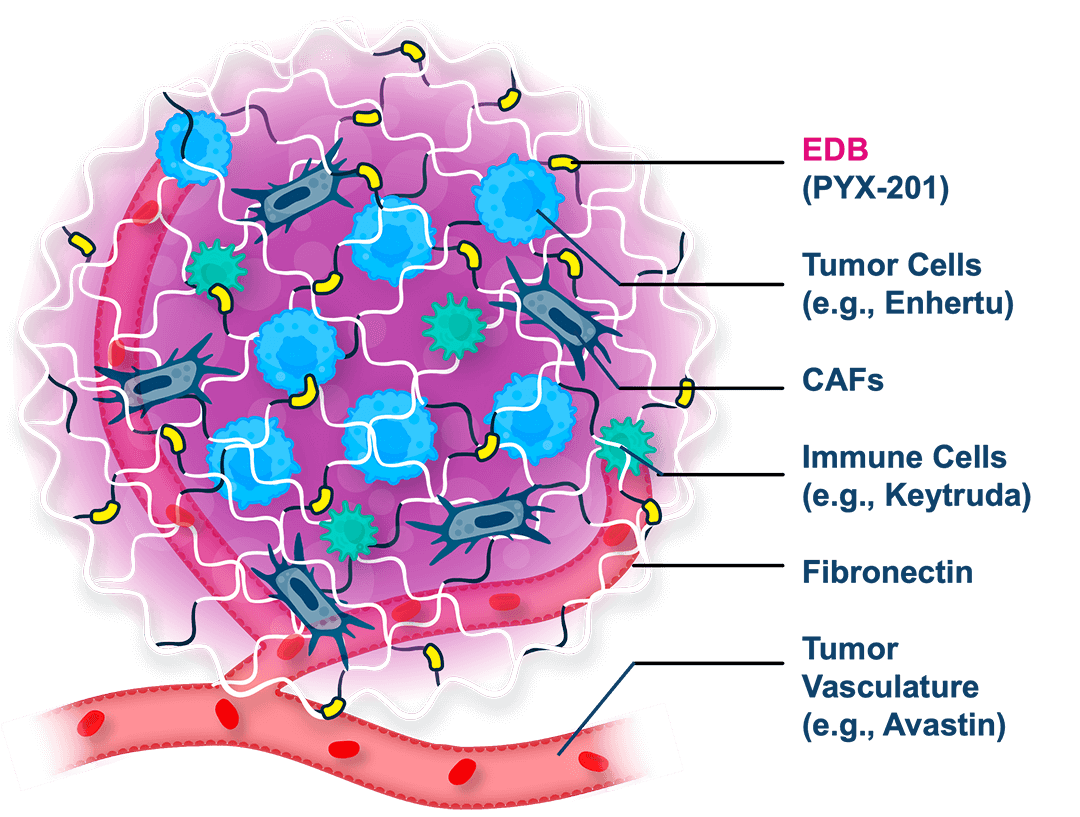
A first-in-concept investigational antibody-drug conjugate (ADC) targeting a noncellular structural component of the extracellular matrix (ECM) with a three-pronged mechanism of action
MICVO is an investigational ADC that uniquely targets the extradomain-B splice variant of fibronectin (EBD+FN), an extracellular matrix protein selectively overexpressed in the tumor microenvironment (TME) across a wide range of solid tumors, but largely absent from normal adult tissues.
The tumor-specific extracellular targeting strategy, along with MICVO’s intentional antibody/linker/payload design, distinguishes MICVO from conventional ADCs by potentially enabling efficient payload delivery across diverse tumor types.
What are ADCs?
ADCs are powerful cancer treatments that combine an antibody with a powerful drug, allowing for targeted delivery directly to cancer cells. This approach offers several advantages over traditional chemotherapy, such as more precise targeting, enhanced effectiveness, and reduced side effects.
With advancements in technology, a new generation of ADCs is being developed, incorporating improvements in key components including conjugation methods, linkers, and drug payloads.
MICVO has the potential to exert anti-tumor activity through three synergistic mechanisms:
Payload driven
1
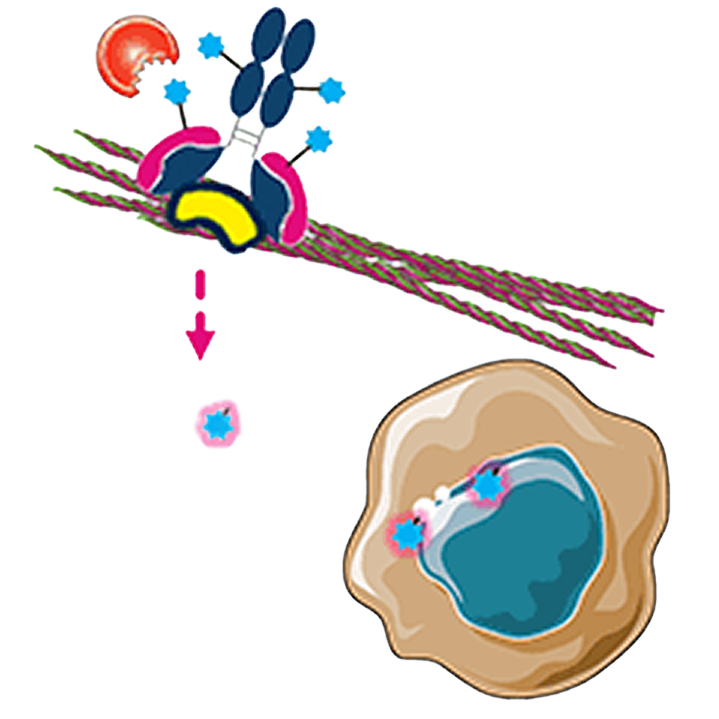
Direct tumor cell killing
MICVO is designed to bind to the EDB+FN target and release payload within tumor ECM
2
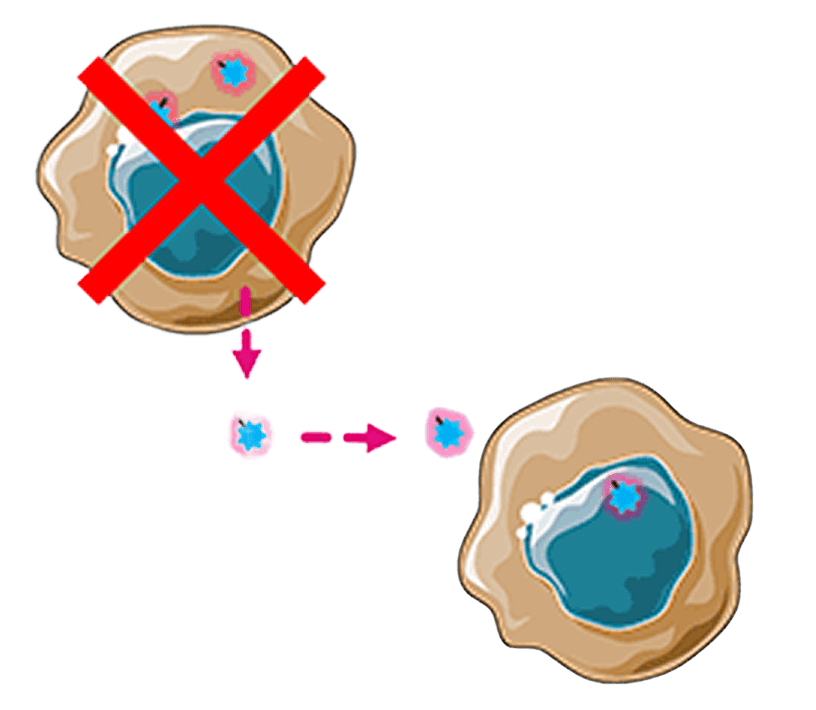
Bystander killing
Payload diffuses into adjacent tumor cells
Tumor cell dies, releasing payload for additional cycles of tumor cell killing
Immune driven
3
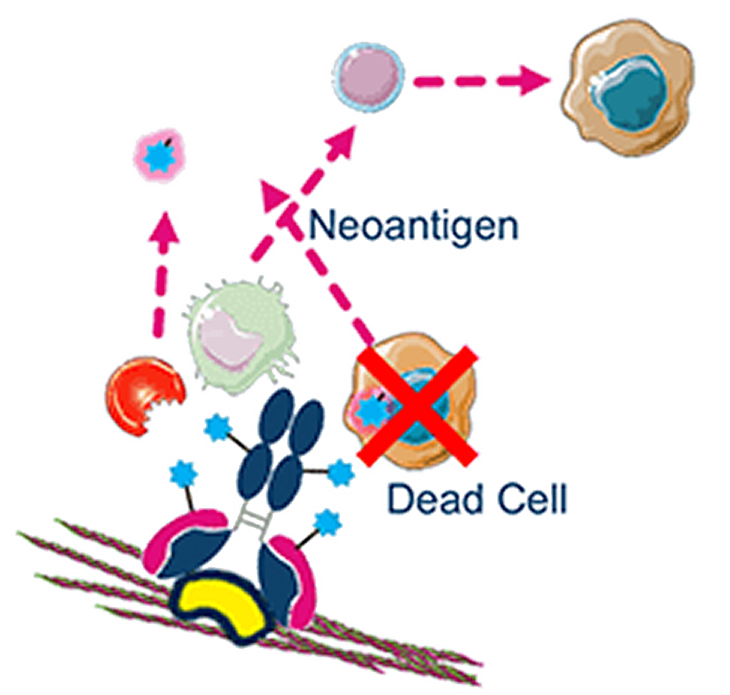
Immunogenic cell death
Host immune activation is triggered, enhancing therapeutic response
Tumor death and exogenous antibody fragments lead to T-cell activation

MICVO also modifies the tumor microenvironment (TME) by eliciting immune cell infiltration which provides a compelling rationale for combination therapies, including for tumors resistant to conventional ADCs.
MICVO: Next steps
Pyxis Oncology is advancing MICVO as a monotherapy (NCT05720117) and in combination with KEYTRUDA® (pembrolizumab) (NCT06795412) across multiple solid tumors with a focus on recurrent/metastatic head and neck squamous cell carcinoma.
Learn about these clinical trials on clinicaltrials.gov:
The U.S. Food and Drug Administration granted Fast Track Designation to MICVO for the treatment of adult patients with R/M HNSCC whose disease has progressed following treatment with platinum-based chemotherapy and an anti-PD-(L)1 therapy.
Pipeline
MICVO - EDB+FN ADC
Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma (R/M HNSCC)
Preclinical
IND-Enabling
Phase 1
Phase 2
Phase 3
MICVO MONO
(Fast track designation granted by FDA)
R/M HNSCC 2L+ Post Platinum & anti-PD(L)-1 Experienced
Pre-Cl.
IND-En.
Ph1
Ph2
Ph3
NEXT MILESTONE Mature Dose Expansion Data Mid 2026
MICVO MONO
R/M HNSCC 2L+ Post EGFRi and/or anti-PD(L)-1 Experienced
Pre-Cl.
IND-En.
Ph1
Ph2
Ph3
NEXT MILESTONE Mature Dose Expansion Data Mid 2026
MICVO + *KEYTRUDA Combo
R/M HNSCC 1/2L+
Additional Tumor Types
Pre-Cl.
IND-En.
Ph1
Ph2
Ph3
NEXT MILESTONE Updated Dose Escalation Data 2H 2026
Combo Therapy Dose Escalation (Various Solid Tumors)
Preclinical
IND-Enabling
Phase 1
Phase 2
Phase 3
MICVO + *KEYTRUDA Combo Dose Escalation
Various solid tumors
Pre-Cl.
IND-En.
Ph1
Ph2
Ph3
Combo dose selection mid-2025
*KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Scientific publications
Learn more about Pyxis Oncology’s clinical pipeline and scientific leadership.
Micvotabart pelidotin (MICVO, formerly PYX-201) is not approved and its safety and effectiveness have not been established.